Science
In reply to the discussion: Marie Curie's notebooks are still radioactive and will be for more than a millennium. [View all]NNadir
(37,535 posts)...below with half-lives included:
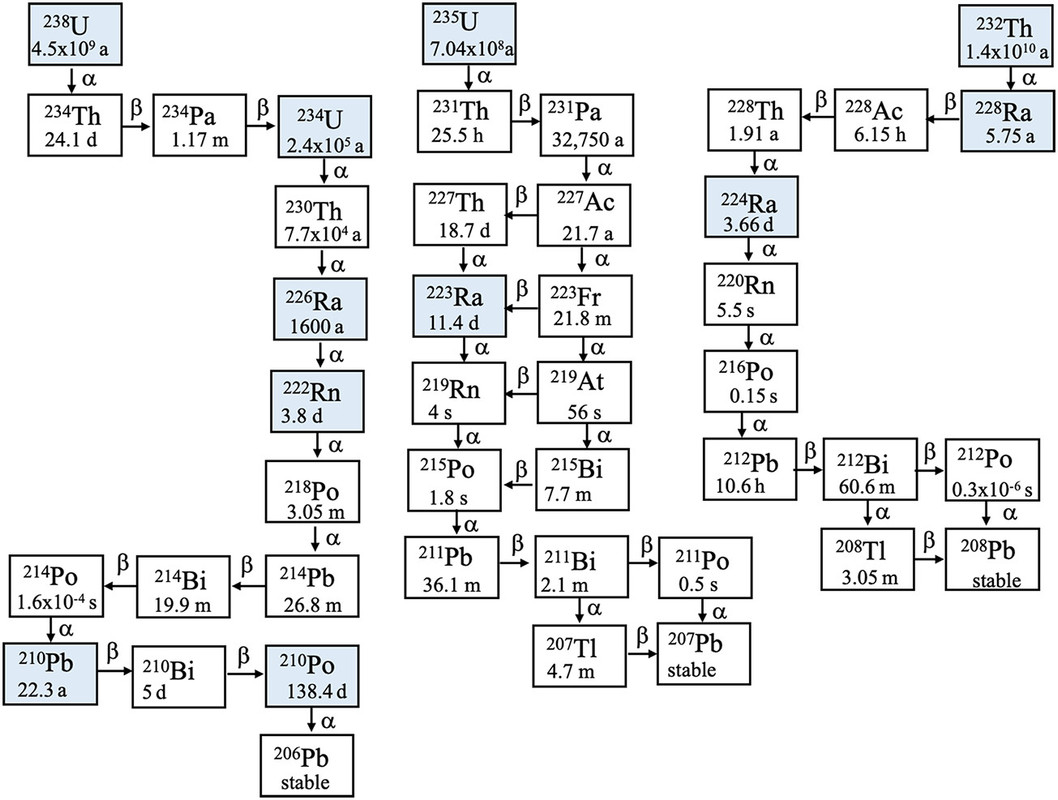
It begins in the chain on the left, 238U at 226Ra, the only isotope of radium that can be isolated in visible amounts. (The half-life in the diagram, 1600 years, is involved in the definition of the nonstandard unit, still in use, the Curie, which is now defined as exactly 3.7 X 1010 nuclear decays per second per gram.
The other two chains shown also occur on Earth, the 235U decay chain, which gives us access to nuclear power, and the 232Th decay chain. There is also a 4th decay chain, that probably once was present on Earth but is now extinct, the 237 Np ( 247Cf) decay chain. This chain has been reestablished on Earth via the use of nuclear power, since 237Np is formed in nuclear reactors, where, when isolated, it has been used to make 238Pu for space missions like the Voyagers, Cassini and Galileo and others. Neptunium can also be used, when enough has been accumulated, as an excellent nuclear fuel where it will be valuable to denature plutonium making it useless for nuclear weapons.
All of the radioactive isotopes below 226Ra are present in Dr. Skłodowska Curie's notebooks and other objects she used during her life, except for 222Rn, which is a gas, representing the largest natural cause of lung cancer, which until people began to smoke tobacco, was a very rare disease. (The parent, uranium, is widely distributed on Earth in vast quantities, notably in most granite, basalt and seawater. It is the major source of heat in the Earth's mantle and inner cores.)
Because 226Ra is a gas, its decay products, shown in the diagram, will be deposited on the walls of the lead containers in which the notebooks and objects are stored. All are highly radioactive with the exception of the stable end nuclide 206Pb. (Much of the lead on Earth started out as uranium.)
All of these decay products have reached "secular equilibrium" which is the point at which the "daughter" nuclei are decaying as fast as they are formed. This situation is also present in so called "nuclear waste," used nuclear fuel; unlike dangerous fossil fuel waste, which, opposed to so called "nuclear waste" actually kills people in vast amounts, fission products cannot accumulate indefinitely. There is a maximum quantity of fission products that can accumulate, depending on the power level of all the nuclear plants operating on Earth, before all of the fission and activation products in them, will decay as fast as they are formed, which can be shown to be asymptotically reached in time. This is why, after a few hundred years in the case where the plutonium and the transplutonium elements are put to use as fuel, that nuclear power will reduce the radioactivity of the planet as a whole, which may or may not be a good thing.
The major radioactive decay products present in the notebooks are those with the longest half-lives a radioactive isotope of lead, 210Pb and 210Po, an isotope of polonium. Polonium was also discovered by Dr. Skłodowska Curie, which she named after her home country, Poland. She was not able to isolate it in visible amounts, but detected it via its radioactive decay and its chemistry. 210Po is the only radioisotope that has ever been utilized in the much hyped and feared case of "nuclear terrorism," having killed one person, Alexander Litvinenko, killed at the behest of the terrorist Vladmir Putin, the person who owns the orange pedophile in the White House. Russia is the world supplier of industrial 210Po which has a number of technological uses chiefly in the elimination of static in the manufacture of products like semiconductors, which are increasingly manufactured on a micro or nano scale.
By using 210Po to kill Litvinenko, the terrorist Putin was able to satisfy the requirement for revenge set out by Edgar Allen Poe in the famous story The Cask of Amontillado, that the person who gets his revenge must be known to the subject of the vengeance. In using this method Putin was able to induce fear in anyone who might seek to repeat Litvinenko's actions.
Thanks for your interest. I hope this helps.
Have a nice weekend.
